Joule's Law
Joule's law stated as the change in internal energy of an ideal gas with volume at constant temperature is zero.
 where, dU and dV are the change in internal energy and change in volume respectively.
where, dU and dV are the change in internal energy and change in volume respectively.
This rule has been derived from Joule Thomson experiment as described below-
A combination of bulb A and B connected through a stopcock 'S' is immersed in water taken in a trough as shown below in figure. Bulb A contains an ideal gas at a certain pressure with the stopcock closed. There is complete vaccum in bulb B. The bulbs reach in a thermal equilibrium after some time. The temperature of water is noted.
The stopcock is now opened and the gas is allowed to move to the bulb B and after some time, bulbs reach in a thermal equilibrium. The temperature of water is again noted.

We find that there is no change in temperature. On opening the stopcock, the gas from the bulb A expands into the evacuated bulb B
i.e. the opposing force Popposing = 0.
Thus, work of expansion-
dW = − PopposingdV = 0
From the first law of thermodynamics,
dU = dq + dw
or, dU = dq (As, dw = 0)
As the temperature of water is not changed
dq = 0
Hence, dU = 0
The mathematical form of Joule's law is derived as under-
Taking U as a function of volume and temperature,
So, U = f (V,T)
Differentiating the equation we get-
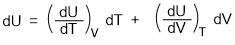
We found earlier that in such situation
dU = 0 and dT = 0
So, the above equation becomes-
(dU/dV)T.dV = 0
but dV ≠ 0
Therefore, (dU/dV)T = 0
Souce: Physical Chemistry by R.L.Madan
