Alcohols Phenols and Ethers Notes
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary alcohols only), identification of primary, secondary and tertiary alcohols, mechanism of dehydration, and uses with special reference to methanol and ethanol.
Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of phenol, electrophilic substitution reactions, uses of phenols.
Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses.
Alcohols are a class of organic in which at least one hydroxyl groups (-OH) attached to a carbon atom. Their general formula is Cn H2n+1 OH or R − OH, where, n is number of carbon atoms and R is an alkyl or a substituted alkyl group. Alcohols are used as solvents, beverages, antiseptics, fuels, perfumes, cleaning agents etc.
Methods of Preparation of Alcohols
From Grignard Reagent (RMgX)
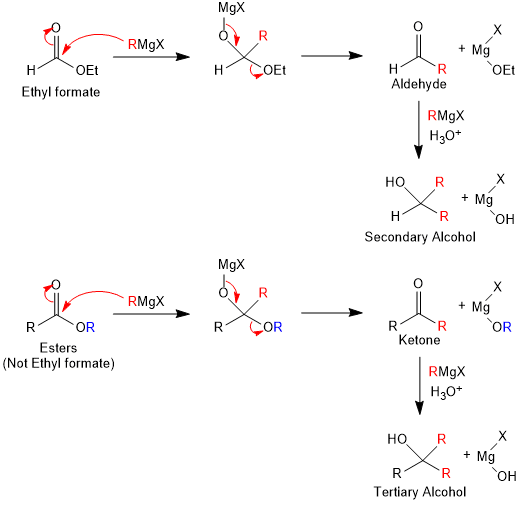
Primary, secondary and tertiary alcohols can be prepared by using Grignard reagent. Grignard reagent reacts with carbonyl compounds to form an intermediate compound, which on hydrolysis, gives alcohols. Formaldehyde when reacted with Grignard reagent gives primary alcohol while the other aldehydes give secondary alcohols. Ketones give tertiary alcohols.

Grignard reagent also reacts with oxirane (Epoxy ethane or Ethylene oxide) to give alcohols
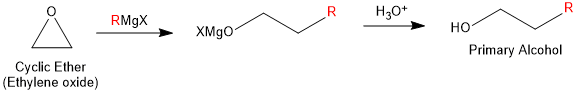
Grignard reagent on reaction with Ethyl formate gives 2°-alcohols and with other esters give 3°-alcohols.
From Carbonyl Compounds
By Reduction of Aldehydes and Ketones
Aldehydes gives primary alcohols while Ketones give secondary alcohols on reduction by reducing agents like LiAlH4, NaBH4, Zn/HCl or H2/Ni.

By Reduction of Carboxylic Acids and Esters
Carboxylic acids are reduced to primary alcohols in excellent yields by lithium aluminium hydride, a strong reducing agent.

Commercially, acids are reduced to alcohols by converting them to the esters followed by their reduction using hydrogen in the presence of catalyst (catalytic hydrogenation).
In case a >C=O group is also present along with –COOH or –COOR groups then use NaBH4 to reduce >C=O group.

From Alkene
Oximercuration and Demercuration of Alkenes
Alkenes undergo oximercuration by mercuric acetate in presence of tetrahydrofuran (THF) and water followed by reduction by NaBH4 (demercuration) to give alcohols according to Markovnikov's rule.

Hydroboration–Oxidation of Alkenes
Diborane (BH3)2 reacts with alkenes to give trialkyl boranes as addition product. This is oxidised to alcohol by hydrogen peroxide in the presence of aqueous sodium hydroxide.

Acid Catalysed Hydration
Alkenes react with water in the presence of acid as catalyst to form alcohols. In case of unsymmetrical alkenes, the addition reaction takes place in accordance with Markovnikov's rule.

From Alkyl Halides
Alcohols can be obtained easily by the hydrolysis of alkyl halide using aqueous KOH.
R – X + KOH(aq) → ROH + KX
Physical Properties of Alcohol
◉ Lower alcohols (ethanol, methanol) are sweet smelling liquids and colourless.
◉ Higher alcohols are odourless, colourless, waxy solids.
◉ Due to formation of intermolecular Hydrogen bonding with water molecules, alcohols are highly soluble in water when present in any proportion.
◉ Boiling points of alcohols are greater than alkyl halides or corresponding ethers due to presence of intermolecular hydrogen bonding in alcohols.
Chemical Properties of Alcohol
Acidic and Basic Behaviour
Alcohol behave both as acids and bases. They are weakly acidic. A strong base such as a hydride ion (H–) in sodium hydride (NaH), can remove the proton from the alcohol molecule and an alkoxide ion results.
R–O–H + B– ⇌ R–O– + BH
Alkoxide ion

Formation of Alkoxides
Alcohols react with sodium or potassium metals to give the respective alkoxides which further react with alkylhalides to form ethers.

Esterification
Alcohols and phenols react with carboxylic acids and acid derivatives (acid chlorides or acid anhydrides) to form esters. This reaction is called esterification reaction and is reversible in nature.

Reaction with Grignard Reagent
When alcohols react with Grignard reagents, an acid-base reaction occurs that produces alkanes and magnesium alkoxide.
R–OH + R–Mg–X → R–H + RO–Mg–X
Reaction with Hydrogen Halides (Groove's process)
Alcohols react with hydrogen halides to form alkyl halides (see haloalkanes and haoarenes).
R–OH + H–X → R–X + H2O
The difference in reactivity of three classes of alcohols with HCl distinguishes them from one another (Lucas test). Alcohols are soluble in Lucas reagent (conc. HCl and ZnCl2) while their halides are immiscible and produce turbidity in solution. In case of tertiary alcohols, turbidity is produced immediately as they form the halides easily. Primary alcohols do not produce turbidity at room temperature.
Reaction with Phosphorus Halides and Thionyl Chloride
Alcohols are converted to alkyl halides by reaction with phosphorus halides and thionyl chlorides (see haloalkanes and haoarenes).
Dehydration of Alcohols
Alcohols undergo dehydration to form alkenes on treating with a protic acid e.g.,concentrated H2SO4 or H3PO4, or catalysts such as anhydrous zinc chloride or alumina. Secondary and tertiary alcohols are dehydrated under milder conditions.

At 100°C, ethyl alcohol reacts with concentrated H2SO4 to form ethyl hydrogen sulfate.
C2H5–OH + H2SO4 → C2H5HSO4 + H2O
At 140°C, ethyl alcohol reacts with concentrated H2SO4 to form diethyl ether.
C2H5HSO4 + C2H5–OH → C2H5–O–C2H5 + H2SO4
At 170°C, ethyl alcohol reacts with concentrated H2SO4 to form ethene.
C2H5–OH + H2SO4 → CH2=CH2 + H2O
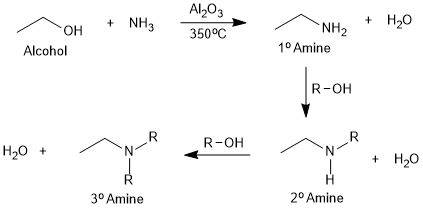
Reaction with Ammonia (NH3)
When an alcohol reacts with NH3 over heated alumina, a primary amine is obtained along with 2° and 3° amines.
Oxidation of Alcohols
Oxidation of alcohols involves the formation of a carbono-xygen double bond with cleavage of an O–H and C–H bonds.
Strong oxidising agents such as acidified potassium permanganate are used for getting carboxylic acids from alcohols directly.
Primary alcohols are oxidized to aldehydes with pyridinium chlorochromate (PCC) and with K2Cr2O7 or KMnO4 give acids which have the same number of carbon atoms, as the parent alcohol.

Secondary alcohols are oxidized to ketones by chromic anhyride (CrO3) which on further oxidation give acids with one carbon atom less than the parent alcohol under drastic conditions by strong oxidizing agents like HNO3.

Tertiary alcohols do not undergo oxidation reaction. However, they forms an acid with two carbon atom less than the parent alcohol by oxidizing under drastic conditions by strong oxidizing agents (KMnO4) at elevated temperatures.

Dehydrogenation of Alcohols with Cu
When the vapours of a primary or a secondary alcohol are passed over heated copper at 573 K, dehydrogenation takes place and an aldehyde or a ketone is formed while tertiary alcohols undergo dehydration. It is used as a test for the detection of all three alcohols i.e. primary, secondary and tertiary.

Reaction of Alcohols with Carbonyl Compounds
An alcohol reacts with a carbonyl compound to form Ketal or acetal. This follows a nucleophilic addition reaction.

Distinction between Primary, Secondary and Tertiary Alcohols
Lucas test
Victor Mayer Test
More tests Tests for Alcohols
Phenols are a class of organic compounds in which a hydroxyl group (-OH) is directly bonded to an aromatic hydrocarbon ring. At room temperature, phenols appear either as colourless liquids or white solids. Phenol is used as antiseptic, disinfectant, reagent, in medicines, in the manufacturing of Bakelite. It is also known as carbolic acid.
Methods of Preparation of Phenol
From chlorobenzene (Dow's process): Lab method
Phenol can be prepared by Dow's process which involves the hydrolysis of chlorobenzene with aqueous NaOH at high temperature and pressure followed by treatment with dilute HCl.


IIT-JEE(Main) Shift-1 Date:23.1.2025
In the following reaction sequence find the product A and B

Solution:
In the first step chlorobenzene is converted into phenol by Dow's process followed by oxidation in acidic medium by potassium dichromate to benzoquinone in the second step.

From Cumene (Cumene process)
In this process, benzene is first converted into cumene with propene then oxidized by air to cumene hydroperoide followed by hydrolysis.

From Benzene Diazonium salt
A diazonium salt is formed by treating an aromatic primary amine with nitrous acid at 273-278 K. Diazonium salts are hydrolysed to phenols by warming with water or by treating with dilute acids.

Physical Properties of Phenol
Boiling point of Phenol: Phenol has generally higher boiling point compared to other hydrocarbons of the same molecular weight because, there are intermolecular hydrogen bonds between the hydroxyl groups of the phenol molecule.
Solubility of Phenol: The solubility of phenol in water depends on the hydroxyl groups present. The hydroxyl groups of phenol are involved in the formation of intermolecular hydrogen bonds. Hydrogen bonds are formed between water and phenol molecules, making phenol water-soluble.

However, the aryl group attached to the hydroxyl group is hydrophobic in nature. Therefore, as the size of the aryl group increases, the solubility of phenol decreases. The solubility of phenols increase with increase in the acidic strength. It is enhanced when -I and -M groups (like -Cl, -Br, -NO2) are introduced on the ring particularly at ortho and para positions. These groups reduces the negative charge on the phenoxide ion and stabilize it.
Acidity of Phenol: Phenol reacts with active metals such as sodium and potassium to form the corresponding phenoxide. The formation of phenoxide ion show the acidic nature of phenol. In phenol, the sp2 hybrid carbon of the benzene ring is directly attached to the hydroxyl group and functions as an electron-withdrawing group. Therefore, the electron density of oxygen decreases.

Phenoxide ions are more stable than alkoxide ions because the negative charge of the oxygen is delocalized over the benzene ring. As a result, phenol is more acidic than alcohol. In substituted phenols, the acidity decreases when an electron-donating group is attached to the ring, and increases when an electron-withdrawing group is attached.

Chemical Properties of Phenol
Electrophilic Substitution Reaction
The –OH group attached to the benzene ring activates towards electrophilic substitution and directs the incoming group to ortho and para positions in the ring as these positions become electron rich due to the resonance effect caused by –OH group.
Nitration of Phenol
With dilute nitric acid at low temperature (298 K), phenol yields a mixture of ortho and para nitrophenols.

The ortho and para isomers can be separated by steam distillation. o-Nitrophenol is steam volatile due to intramolecular hydrogen bonding while p-nitrophenol is less volatile due to intermolecular hydrogen bonding which causes the association of molecules.

With concentrated nitric acid, phenol is converted to 2,4,6-trinitrophenol. The product is commonly known as picric acid. The yield of the reaction product is poor.

How picric acid get in high yield from phenol?
Picric acid is prepared by treating phenol first with concentrated sulphuric acid which converts it to phenol-2,4-disulphonic acid, and then with concentrated nitric acid to get 2,4,6-trinitrophenol.

Halogenation of Phenol
On treating phenol with bromine, different reaction products are formed under different experimental conditions.
When the reaction is carried out in solvents of low polarity (CHCl3) or in non-polar solvent (CS2) at low temperature, monobromophenols are formed.
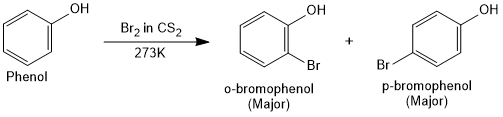
When phenol is treated with bromine water (polar solvent), 2,4,6-tribromophenol is formed as white precipitate.


IIT-JEE(Main) Shift-1 Date:23.1.2025
If 2 g phenol is allowed to react with Br2/H2O. How much Br2 (in g) will be required to produce 2, 4, 6 tribromophenol (Rounded off to nearest integer).
Solution:
3 moles Br2 will be required to react with 1 mole phenol.
Molecular weight of phenol = (12 × 6) + 6 + 16 = 94
Molecular weight of 3 moles of Br2 = 3 × 160 = 480
∵ 94 of phenol requires = 480 g of Br2
∴ 2 g of phenol requires = (480 × 2)/94 = 10.22 g
Kolbe's Reaction
Kolbe's reaction, also known as Kolbe-Schmitt reaction. Phenoxide ion generated by treating phenol with sodium hydroxide is even more reactive than phenol towards electrophilic aromatic substitution. Hence, it undergoes electrophilic substitution with carbon dioxide, a weak electrophile. Ortho hydroxybenzoic acid is formed as the main reaction product. Simply we can say that Phenoxide ion reacts with carbon dioxide under pressure followed by acidification yield salicylic acid. This is Kolbe's reaction.

Reimer-Tiemann Reaction
On treating phenol with chloroform in the presence of sodium hydroxide, a formyl (–CHO) group is introduced at ortho position of benzene ring. This reaction is known as Reimer Tieman reaction. The intermediate substituted benzal chloride is hydrolysed in the presence of alkali to produce salicylaldehyde.

Read Reimer Tieman Reaction in details.

IIT-JEE(Main) Shift-2 Date:30.1.2024
Which reagent on reacting with phenol gives salicylaldehyde?
A. CO2, NaOH
B. CHCl3, NaOH
C. CCl4, NaOH
D. H2O, H+
Solution:
Salicylaldehyde is produced when phenol reacts with chloroform and sodium hydroxide followed by acidification. This reaction is called the Reimer-Tiemann reaction.
IIT-JEE(Main) Shift-1 Date:30.1.2024
What happens when phenol is treated with chloroform in presence of NaOH at 343K followed by hydrolysis ?
A. Salicylic Acid
B. Salicylaldehyde
C. Benzaldehyde
D. Benzoic acid
Solution:
Salicylaldehyde is produced when phenol reacts with chloroform and sodium hydroxide followed by acidification. This reaction is called the Reimer-Tiemann reaction.
Reaction of Phenol with Zinc Dust
Phenol is reduced to benzene on heating with zinc dust. In this reaction, zinc acts as a reducing agent which reduce the phenol to benzene while the zinc is oxidized to zinc oxide (ZnO).

Oxidation of Phenol
Oxidation of phenol with chromic acid produces a conjugated diketone known as benzoquinone. In the presence of air, phenols are slowly oxidised to dark coloured mixtures containing quinones.
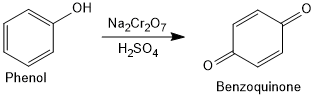
In the presence of mild oxidizing agents, phenol can be converted to catechol (1,2-dihydroxybenzene). Phenol can also be oxidized to hydroquinone (1,4-dihydroxybenzene), depending on the specific oxidizing agents and reaction conditions.
Acylation of Phenols (Friedel-Crafts Acylation)
Phenols are examples of bidentate nucleophiles, meaning that they can react at two positions:
on the aromatic ring giving an aryl ketone via C-acylation, a Friedel-Crafts reaction or
on the phenolic oxygen giving an ester via O-acylation, an esterification.

The product of C-acylation is more stable and predominates under conditions of thermodynamic control (i.e. when AlCl3 is present). The product of O-acylation forms faster and predominates under conditions of kinetic control.
Aryl esters readily rearrange to aryl ketones in the presence of AlCl3, a reaction known as the Fries rearrangement as shown above (O-acylation to o- and p-aryl ketones).
Alylation of Phenols (Friedel-Crafts Alkylation)
Phenol can undergo Friedel-Crafts alkylation by using a reagents that can generate electrophile without the use of Lewis acids.

Reaction of Phenol with Formaldehyde
Phenol and formaldehyde react to form phenol-formaldehyde resins, also known as phenolic resins or phenoplasts. This reaction is a condensation reaction that produces synthetic polymers.
Read Lederer-Manasse Reaction
Gattermann Reaction
Phenol reacts with the mixture of HCN and HCl in the presence of ZnCl2 catalyst aldimine formed as intermediate which on hydrolysis forms p-hydroxybenzaldehyde. This reaction is known as Gattermann reaction.

Reaction with Benzene diazonium Chloride
Phenol couples with benzene diazonium chloride in an alkaline solution to form p-hydroxy azobenzene(a red orange dye).

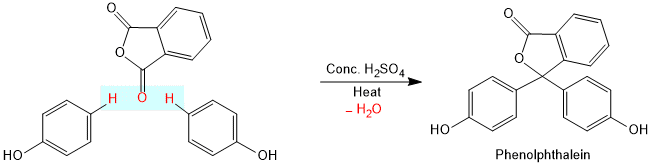
Phthalein Reaction
On heating phenol (2 molecules) with phthalic anhydride in presence of con. H2SO4, phenolphthalein is obtained.
Tests of Phenol
Ferric Chloride Test
Aqueous solution of phenol reacts with freshly prepared ferric chloride solution gives coloured complex.

Libermann's Test
Phenol reacts with concentrated sulfuric acid and sodium nitrite forms a yellow colour quinone monoxime complex. With excess of phenol and sulfuric acid a deep blue indophenol complex is formed. On dilution a red colour indophenol is formed which turns to deep blue colour sodium salt solution of indophenol on treatment with sodium hydroxide.

Bromine Water Test
Phenol undergoes electrophilic substitution reaction with bromine. When bromine water is added to aqueous solution of phenol the brown colour of bromine disappears and a white precipitate of tribromophenol is formed.

Phthalein Dye Test
Phenol on heating with phthalic anhydride in the presence of concentrated sulfuric acid forms a colourless condensation compound called phenolphthalein. On further reaction with dilute sodium hydroxide solution gives a pink colour. This is called phthalein dye test.

Ethers are a class of organic compounds that contains an oxygen atom connected by single bond to two alkyl or aryl groups. Their general formula is R − O − R′ , where R and R′ may be same or different. These compounds are used in dyes, perfumes, oils, waxes and industrial uses. Diethyl ether is widely use as an anesthetic in the past.
Methods of Preparation of Ethers
Williumson Synthesis
It is an important laboratory method for the preparation of symmetrical and unsymmetrical ethers. In this method, an alkyl halide is allowed to react with sodium alkoxide.
R–X + R–ONa → R–O–R + Na–X
Ethers containing substituted alkyl groups (secondary or tertiary) may also be prepared by this method. The reaction involves SN2 attack of an alkoxide ion on primary alkyl halide.
Better results are obtained if the alkyl halide is primary. In case of secondary and tertiary alkyl halides, elimination competes over substitution. If a tertiary alkyl halide is used, an alkene is the only reaction product and no ether is formed. For example, the reaction of CH3ONa with (CH3)3C–Br gives exclusively 2-methylpropene

It is because alkoxides are not only nucleophiles but a strong bases as well. They react with alkyl halides leading to elimination reactions.
By Dehydration of Alcohols
Alcohols undergo dehydration in the presence of protic acids (H2SO4, H3PO4) to form ethers at 443K.
C2H5–OH → C2H5–O–C2H5
The method is suitable for the preparation of ethers having primary alkyl groups only otherwise the reaction favours the formation of alkene by follows SN1 pathway.

IIT-JEE(Main) Shift-1 Date:22.1.2025
Statement-I: CH3–O–CH2–Cl will show nucleophilic substitution by SN1 mechanism in protic medium.
Statement-II: (CH3)3C–CH2–Cl will not undergo nucleophilic substitution via SN2 mechanism easily.
A. Statement-I and statement-II both are correct
B. Statement-I and statement-II both are incorrect
C. Statement-I is correct but statement-II is incorrect
D. Statement-I is incorrect but statement-II is correct
Solution:
A is the correct option.
CH3–O–CH2+ is stabilised by resonance.
Synthesis of Methoxy Ethers
Methoxy ethers can be easily prepared by the reaction of alcohol with diazomethane in the presence of HBF4.
R–OH + CH2N2 → R–O–CH3 + N2↑
Physical Properties of Ethers
◉ Dimethyl ether and diethyl ether are solids and rest are liquids. Some aromatic ethers are solid.
◉ Ethers have a lower boiling points than their corresponding isomeric alcohols, as they do not from hydrogen bond like alcohols. For example,
C2H5OH > CH3–O–CH3.
◉ Ethers are partially soluble in water due to the formation of hydrogen bonds with water.
◉ Ethers are weak Bronsted bases or Lewis bases, as the central atom oxygen has 2 lone pair of electrons to donate and can also accept H+
ions.
◉ Due to presence of lone pair of electrons on oxygen atom, ethers have some value of dipole moment.
Chemical Properties of Ethers
Cleavage of C–O Bond in Ethers
Ethers are the least reactive like alkanes. The cleavage of C-O bond in ethers takes place under drastic conditions with excess
of hydrogen halides. The reaction of dialkyl ether gives two alkyl halide molecules.
R–O–R + H–X → R–X + R–OH
R–OH + H–X → R–X + H2O
Ethers with two different alkyl groups are also cleaved in the same manner.
R–O–R + H–X → R–X + R–OH
The order of reactivity of hydrogen halides is as follows:
HI > HBr > HCl.
The cleavage of ethers takes place with concentrated HI or HBr at high temperature.
When one of the alkyl group is a tertiary group, the halide formed is a tertiary halide because, in step 2 of the reaction, the departure of leaving group (HO–CH3) creates a more stable carbocation [(CH3)3C+], and the reaction follows SN1 mechanism.

In case of anisole, methylphenyl oxonium ion, is formed by protonation of ether. The bond between O–CH3 is weaker than the bond between O–C6H5 because the carbon of phenyl group is sp2 hybridised and there is a partial double bond character. Therefore, the attack by I– ion breaks O–CH3 bond to form CH3I. Phenols do not react further to give halides because, the sp2 hybridised carbon of phenol cannot undergo nucleophilic substitution reaction needed for conversion to the halide.

Alkyl aryl ethers are cleaved at the alkyl-oxygen bond due to the more stable aryl-oxygen bond. The reaction yields phenol and alkyl halide but not aryl halide and alkyl alcohol.


IIT-JEE(Main) Shift-2 Date:29.1.2025
Which of the following ether react with HBr to form phenol?
A. Ph − CH2 − O − CH2 − CH3
B. Ph − CH2 − O − CH3
C. Ph − CH2 − O − CH2 − Ph
D. Ph − O − CMe3
Solution:
Option D is correct answer because phenoxide ion is more stable than benzyl cation.
Electrophilic Substitution Reactions
The alkoxy group (-OR) is ortho, para directing and activates the aromatic ring towards electrophilic substitution in the same way as in phenol.
Halogenation of Anisole
Anisole undergoes bromination with bromine in ethanoic acid even in the absence of iron (III) bromide catalyst due to the activation of benzene ring by the methoxy group. Para isomer is obtained in 90% yield.

Friedel-Crafts Reaction of Anisole
Anisole undergoes Friedel-Crafts reaction, i.e., the alkyl and acyl groups are introduced at ortho and para positions by reaction with alkyl halide and acyl halide in the presence of anhydrous aluminium chloride (a Lewis acid) as catalyst.

Nitration of Anisole
Anisole reacts with a mixture of concentrated sulphuric and nitric acids to yield a mixture of ortho and para nitroanisole.

Reaction with Carbonmonoxide
Ethers reacts with CO to form esters in BF3 at 150° under pressure.
R–O–R + CO → R–CO–O–R
Reaction with PCl5
Ethers on heating with PCl5 gives alkyl chloride.
R–O–R + PCl5 → 2R–Cl + 2POCl3
Questions Answer
How would you convert benzene to phenol via Friedel-Crafts alkylation?
Hint:Benzene → Cumene → Phenol
See methods of preparation of Phenol
How will you convert phenol to aspirin and oil of wintergreen?

Which reagent use to convert the followig reaction?

The oxidation of secondary alcohol to a ketone can be easily achieved by using oxidizing agents like Pyridinium chlorochromate (PCC), chromic anhydride (CrO3) and MnO2 etc.
Explain why p-nitrophenol is more acidic than phenol?
Nitro group of phenol produces – I and – R effect. Because of these two effects —NO2 group is electron withdrawing in nature. So, the electron density in the O—H bond of p-nitrophenol decreases relative to the O—H bond of phenol.
The decrease in electron density of the O—H bond of p-nitrophenol, the polarity of O—H bond is decrease and in turn make it more acidic than phenol.
Explain why alcohols and ethers of comparable molecular mass have different boiling points?
Boiling point depends upon the strength of intermolecular forces of attraction. Higher these forces of attraction, more will be the boiling point. Alcohols undergo intermolecular hydrogen bonding. So, the molecules of alcohols are held together by strong intermolecular forces of attraction.
But in ethers no hydrogen atom is bonded to oxygen. Therefore, ethers are held together by weak dipole-dipole forces, not by strong hydrogen bond. Since, lesser amount of energy is required than to break weak dipole-dipole forces in ethers than to break strong hydrogen bonds in alcohol.
Explain why low molecular mass alcohols are soluble in water?
Solubility of alcohols and phenols in water is due to their ability to form hydrogen bonds with water molecule. The hydrocarbon part methoxy methane (i.e., R group) tends to prevent the formation of hydrogen bonds. Alcohols with lower molar mass will have smaller hydrocarbon part and therefore tendency to form hydrogen bonding is more and they are more soluble in water.
CH3CH2OH > CH3CH2CH2OH
(More soluble) (Less soluble)
Why is the reactivity of all the three classes of alcohols with conc. HCl and ZnCl2 (Lucas reagent) different?
The reaction of alcohols with Lucas reagent (conc. HCl and ZnCl2) follow SN1 mechanism. SN1 mechanism depends upon the stability of carbocations (intermediate). More stable the intermediate carbocation, more reactive is the alcohol.
Tertiary carbocations are most stable among the three classes of carbocations and the order of the stability of carbocation is 3° > 2° > 1°. This order, inturn, reflects the order of reactivity of three classes of alcohols i.e., 3° > 2° > 1°.
Thus, as the stability of carbocations are different so the reactivity of all the three classes of alcohols with Lucas reagent is different.
Explain why nucleophilic substitution reactions are not very common in phenols?
Resonance is an important factor in phenols. During resonance -OH group in phenol gives its electrons to the benzene ring. As a result of this, the electron density on benzene ring is very high. This increased electron density repels nucleophiles.
Therefore, nucleophiles cannot attack the benzene ring and phenols usually do not give nucleophilic substitution reaction.
What is denatured alcohol?
Alcohol is used in large quantities in the manufacture of alcoholic liquors. Its continuous use damages the various vital organs. Therefore, to refrain the people from drinking alcohol, heavy excise duty is levied on the sale of alcoholic beverages. But, it is used in various industries as it is a very good solvent.
Therefore, industrial alcohol must be cheap. Thus, to provide cheaper alcohol to industries and to refrain people from drinking alcohol, it is mixed with some copper sulphate, pyridine, methyl alcohol or acetone.
Alcohol is made unfit for drinking by mixing some quantity of any of these substances in it. This is called denatured alcohol.
