Carbocation: Generation, Structure, Reactivity, Reactions, Stability, and Stereochemical Effect
The term carbocation comes from the word carbo which means carbon and cation which mean positively charged ion. So, carbocation is a chemical species in which carbon having three σ bonds and a positive charge. Carbocations have a positive charge and lacks one electron so it is an electron-deficient species (Lewis acid) and acts as an electrophile. This positively charged carbon has only six electrons in its valence shell so it is quite unstable because it does not satisfy the octet rule. Carbocation is also called carbonium ion.
Generation of Carbocation
Carbocations can be formed during organic reactions by heterolysis as intermediates. Common methods of carbocation formation include ionization of alkyl halides, elimination reactions, and rearrangements.
By ionization in polar protic solvents:
R3C-X → R3C+ + X−
By protonation of substrates
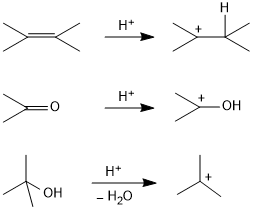
By the decomposition of diazonium salts
RN2Cl → R+ + N2↑ + Cl−
Reactivity of Carbocation
Carbocations are highly reactive due to their electron-deficient nature. They act as electrophiles, seeking to accept a pair of electrons to achieve a more stable configuration. They are involved in various organic reactions, such as nucleophilic attacks, rearrangements, and elimination reactions.
Reactivity order of carbocation: 3° > 2° > 1° > methyl carbocation
Reactions of Carbocation

Structure of Carbocation
Carbon in carbonium ion has three substituents and an empty p-orbital. So, the carbon atom in carbonium ion is sp2 hybridized and it has a trigonal planar geometry.

Stereochemical Effect
The vacant 2p orbital can be attacked from either side of the plane of the ion by an electron-rich species (nucleophile). This may result in the formation of both the optical isomers in suitable substrates.

Stability of Carbocation
Carbocation is an electron deficient species so any effect that reduces positive charge on carbon can stabilize the carbocation. Inductive effect, hyperconjugation and resonance all can do that. These effects are discussed below.
Inductive Effect
Alkyl groups are electron-donating groups (+I Effect). They increase electron density at the carbon having positive charge. This results into the dispersal of positive charge over all the alkyl groups thereby decreasing its electron deficiency, this increase the stability. More the number of alkyl groups attached, more will be the stability of carbocation.
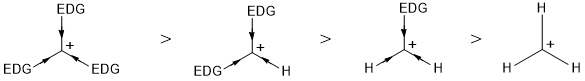

IIT-JEE(Main) Shift-1 Date:22.1.2025
The most stabled carbocation is

Solution:
Option C is correct answer.
-NO2 is EWG which increases the positive charge on methylene group and decreases the stability. -OMe and -OH both are EDG but -OMe is a stronger electron donating group than -OH, meaning that it can better stabilize the positive charge on the benzyl carbon through resonance.

IIT-JEE(Main) Shift-2 Date:22.1.2025
What is correct order of stability of carbocation.

Solution:
C > A > D > B
p-methyl and para-methoxy benzyl carbocation is more stable than an o-methyl and o-methoxy benzyl carbocation.
In both cases, the positive charge on the benzylic carbon can be delocalized through resonance with the benzene ring. However, when the methyl or methoxy group is in the para position, it can directly participate in resonance stabilization by donating electron density through hyperconjugation, making the carbocation more stable compared to the ortho position.
Hyperconjugation
In carbocations the sigma bond in conjugation with vacant p-orbital on carbon carrying positive charge participates in delocalisation, thus spreading the charge all over the alkyl groups. This effect is known as hyperconjugation or no bond resonance. Larger the number of hyperconjugative structure, greater the stability.


In tert-butyl cation, (CH3)3C+ there are nine C-H sigma bonds which participate in delocalisation. In iso-propyl cation, (CH3)2CH+ six C-H sigma bonds and in ethyl cation, CH3CH2+ three C-H bonds are available. Thus (CH3)3C+ is more stable than (CH3)2CH+ which in turn is more stable than CH3CH2+ due to greater number of contributing hyperconjugative structures in former than the latter.
(CH3)3C+ > (CH3)2CH+ > CH3CH2+
Resonance
Conjugation with multiple bond or lone pair of electron increases the stability of a carbocation. For example, triphenylmethyl cation (Ph3C+) is more stable than diphenylmethyl cation (Ph2CH+) which in turn is more stable than phenylmethyl cation (PhCH2+). This due to greater delocalization of positive charge in case of triphenylmethyl cation as it has more number of resonating structures.

If a heteroatom with an unshared pair of electrons is adjacent to the cationic centre then it stabilises the positive charge. Thus the carbocation with positive centre adjacent to the oxygen atom with lone pair of electron is more stable. The lone pair is involved in resonance decrease the electron deficiency of the positive carbon providing it stability.

Also read Non-Classical Carbocations
Which of the following carbocation is most stable?

Answer: Tropolium cation is exceptionally stable due to aromatic character. It is also stabilized by delocalized positive charge across its ring system due to resonance.
Order of stability: D > B > C > A
