Henderson-Hasselbalch Equation: Derivation, Limitations and Applications
Derivation of Henderson-Hasselbalch Equation
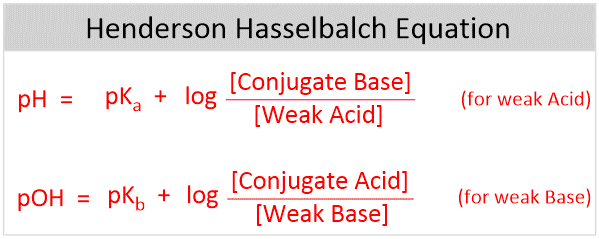
Henderson-Hasselbalch equation is used to determine the pH of a buffer system and can be derived from the Ka of a weak acid.
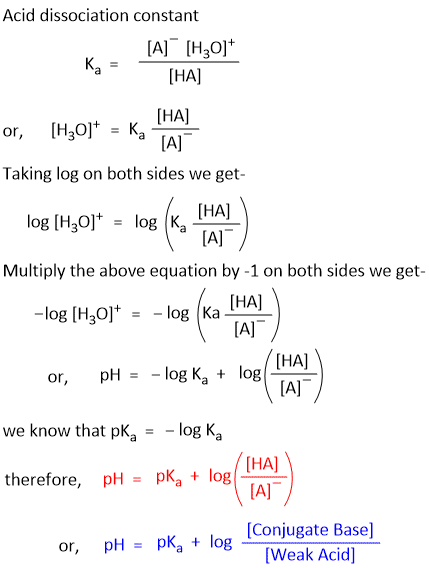
Let us assume a weak acid (HA) and its conjugate base (A–). In water the following dissociation occurs-
HA + H2 ⇌ A– + H3–
According to the law of dissociation, the acid dissociation constant (Ka) can be defined by the equation
Similarly, we can derive the equation for Base-
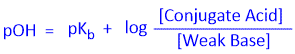
Limitations of Henderson-Hasselbalch Equation
1. Henderson equation fails to explain the accurate values of the strong acids and strong bases.
2. This equation can explain the pH values for very dilute solutions.
3. This equation does not account for the buffer capacity of the solution.
4. This equation is most accurate for solutions where the pH is within one unit of the pKa of the acid.
5. The Henderson-Hasselbalch equation is less straightforward to apply to polyprotic acids.
6. The pKa value can change with temperature, but the Henderson-Hasselbalch equation does not explicitly account for temperature variations
Applications of Henderson-Hasselbalch Equation
There are number of applications of this equation. Some of them are give below-
1. This equation helps to calculate the pH if the ratio of salt to acid is known.
2. It is used to find the change in pH, when the strong base is added to the solution of a weak acid.
3.This equation is used in the determination of the pKa of a weak acid.
4. It is extensively applied in the pharmaceutical industry and drug synthesis
