IPSO Substitution Reaction
IPSO is a Latin word which means on itself. A position which is already occupied by a non-hydrogen substituent in an aromatic ring is called ipso position, the attack on this position is called ipso attack (or ipso addition), and the aromatic substitution in which a substituent already present is replaced is called ipso substitution. The entering group may also itself be expelled or migrate to another position in a subsequent step.
For example, protodealkylation of an alkylbenzene (reverse of Friedel-Crafts alkylation). In this reaction, tertiary alkyl groups are most easily removed, since they depart as stabler carbocations. Thus, t-butyl group is used to protect the most reactive position in a compound to effect reaction elsewhere. The mechanism is given below.

Reangement of alkylbenzenes leading to their isomerization is also involves ipso attack. For example, o-xylene isomerises to m-xylene as follows.

Silyl group has a strong tendency to direct the entering electrophile to the position occupied by it i.e. ipso position. This is due to strong stabilization of cationic center beta to the silicon.
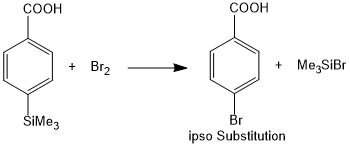

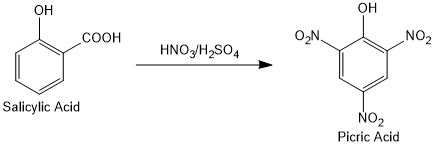
Examples
The reaction of salicylic acid with a mixture of nitric and sulfuric acid to form picric acid is a classical example of ipso substitution reaction.