Non-Classical Carbocation: Generation, Reactivity, Reactions and Stability
Non-classical carbocations may be defined as the carbocations in which the π-bond is not conjugation with the carbon bearing positive charge, and gets stabilized by neighboring group participation by π- or σ-bond.
The term non-classical carbonium ion (carbocation) was first used by J.D. Roberts referring to the tricyclobutonium structure for the cyclopropylcarbinyl cation. Some other examples are 7-norbornenyl cation, norbornyl cation, phenonium ion and cyclopropylmethyl cation.
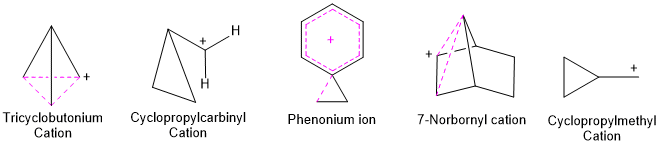
In a nonclassical carbocation, the positive charge is delocalised by a double or triple bond that is not in the allylic position. It is also delocalised by σ-electrons of carbon-carbon or carbon-hydrogen bond or we may say that by a single bond. This means that there are three atoms sharing two electrons in these carbocations (three-center two-electron structure) which may be due to the delocalisation of the electrons. In contrast to a classical carbocation that can be drawn by a single Lewis structure, the non-classical carbocation cannot be drawn by a single Lewis structure.
Like classical carbocations, the carbon in a non-classical carbocation has only six electrons, it is also electron deficient and acts as an electrophile in chemical reactions. Now, whether the neighboring participant is σ-bond or π-bond (not conjugated), non-classical carbocations can be divided into two categories.
Generation of non-classical Carbocations
The generation of non-classical carbocations takes place mainly in neighboring group participation via π- or σ-bond. Some reactions involving the production of non- carbocations are given.
Neighbouring group participation via π-bond
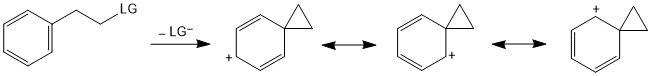
Neighbouring group participation via σ-bond
The aliphatic C–H or C–C bonds can also give rise to delocalization of charge if these bonds are close enough and antiperiplanar to the leaving group. The intermediates corresponding to these mechanisms are nonclassical in nature and the 2-norbornyl system is the most common example.
Acetolysis of exo-2-norbornyl brosylate and endo-2-norbornyl brosylate. In the reactions it was found that the exo-2-norbornyl brosylate was three-hundred and fifty times more reactive than the endo-2-norbornyl brosylate.

Stability of Non-classical Carbocations
The stability of nonclassical carbocations can be understand by the delocalization of σ- or π-bonds as discussed below.
Stability by the delocalization of π-bond: The typical example of this type of stabilization is 2- norbornyl cation which is shown below.

Stability by the delocalization of σ-bond: The treatment of cyclopropylmethyl (or cyclobutyl chloride or homoallyl chloride) chloride with dilute ethyl alcohol yields a mixture of 5% homoallyl alcohol, 47% cyclobutanol, and 48% cyclopropylmethyl alcohol. All this suggests that the carbocationic intermediate present in all three reactions must be the same, which in turn, is responsible for the same resulting products.

Reactivity of non-classical Carbocations
Nucleophilic Attack
A non-classical carbocation may combine with a nucleophile by accepting an electron pair. Furthermore, it should also be noted that if all the three groups on the carbocation are different, a racemic mixture will be obtained.

Rearrangement Reactions
The treatment of cyclopropylmethyl (or cyclobutyl chloride or homoallyl chloride) chloride with dilute ethyl alcohol yields a mixture of 5% homoallyl alcohol, 47% cyclobutanol and 48% cyclopropylmethyl alcohol.

All this suggests that the carbocationic intermediate present in all three reactions must be the same, which in turn, is responsible for the same resulting products.

