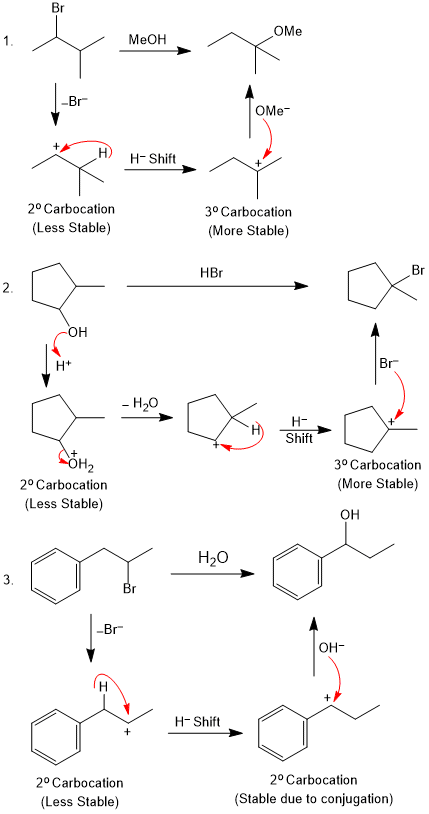
Unimolecular Nucleophilic Substitution (SN1 Reaction)
Nucleophilic substitution reaction that follows 1st order kinetics is called unimolecular nucleophilic substitution (SN1) reaction. The reaction is first order with respect to substrate while zero order with respect to the nucleophile.
Rate = k [Substrate]
SN1 reactions occur in two steps. In the first step, the leaving group (LG) leaves the molecule slowly and generate a carbocation which is rapidlt attacked by nucleophile in the second step, forming the product.

Stereochemistry of SN1 Reaction
Since a carbocation is flat (sp2,trigonal planar), so the attack of the nucleophile can occur from either side of the plane with equal probability. This means that equal amounts of (+) and (-) forms of the product will be produced. The result is racemisation i.e., a racemic product result if the substrate is chiral.

Factors Influencing SN1 Reaction
Solvent: In SN1reactions, ionization occurs in the rate-determining step. Hence, polar solvents promote SN1 reaction.
Nucleophile: In SN1 reaction, a carbocation is attacked by the nucleophile. Hence, a low concentration of weak nucleophile is sufficient for SN1 reaction. A high concentration of strong nucleophile may act as base by accepting proton from suitable carbocation resulting in the formation of alkenes.
Leaving group : Less basic groups are better leaving groups because a strong base has a greater tendency for a backward direction in the reversible reaction. Strong bases such as OH−, OR−, R2N− etc., are not good leaving groups. Thus, alcohols are resistant to substitution in non-acidic medium. In acidic medium, however, the hydroxyl group leaves as H2O, which is a weak base.
Substrate: The hyperconjugation and the inductive effect allow alkyl groups to stabilize carbocations. The more stable carbocation intermediate has a lower activation barrier, so the SN1 reaction occurs faster. In general, the SN1 reaction is favored in the order of
Benzylic > Allylic > 3° > 2° > 1° > Me+
Examples of SN1 Reaction