Bimolecular Nucleophilic Substitution (SN2) Reaction
Nucleophilic substitution reaction in which a nucleophile attacks an electrophile and replaces a leaving group. The reaction proceeds through a concerted mechanism, that means the bond between the nucleophile and the electrophile (LG) is formed at the same time as the bond between the electrophile and the leaving group is broken.

SN2 Reaction follow second-order kinetics that means a concerted one-step reaction without any intermediate. The rate of hydrolysis of methyl bromide with NaOH has been found to be of second order.
Rate ∝ [CH3Br] [OH−]
As the reaction is concerted, the steric factor is very important. Bond making and bond breaking takes place in a single step (transition state has very short time lag), so bulkier substrate does not prefer to undergo SN2 mechanism.

In the transition state, five groups or atoms are bonded to the α-carbon. Increasing the crowding (+I effect) on the carbon bearing the leaving group (LG) decreases the nucleophilic attack because the carbon bearing the leaving group becomes less positively polarised and consequently less readily attacked by the nucleophile.
Therefore, the rate of hydrolysis of alkyl halides by SN2 path is CH3X > 1° > 2° > 3° i.e., reverse of SN1 path.
Stereochemistry of SN2 Reaction
The SN2 reaction is stereospecific like other concerted reactions. Experimental observations show that all SN2 reactions proceed with inversion of configuration (Walden Inversion); that is, the nucleophile will always penetrate from the backside of the leaving group in SN2 reactions.
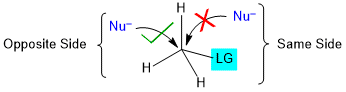
Remember that the stereochemistry is inverted only at the reaction center. Because no bonds are made or broken except at the carbon bonded to the leaving group, the stereochemistry at any other chirality centers in the reactant remains unchanged
.
Factors Affecting the Rate of SN2 Reaction
Substrate: Substrate should be as less hindered as possible because, nucleophile attacks the substrate from the backside, the carbon-LG bond is broken, and the carbon nucleophile bond is formed at the same time.
Nucleophile: Strong nucleophiles are more likely to attack the electrophile and replace the leaving group. Higher concentration of the nucleophile increases the rate of reaction.
Solvent: The role of solvent is very important for the formation of the carbon nucleophile bond. It depends on the solvent and how much hindrance it offers to the flow of nucleophiles to reach the carbon. Polar solvents prefet to SN2 reaction.
Leaving group: Weaker leaving group increases the rate of the reaction. The order of good leaving group is
MsO‒, TsO‒ > I‒ > Br‒ > Cl‒ > F‒ > (OH‒, NH2‒).
Temperature: At the higher temperature, the reaction takes place quickly.
Examples of SN2 Reaction

