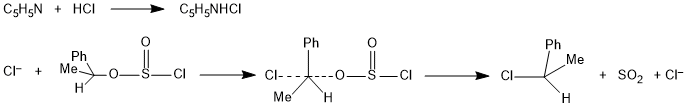
Internal Nucleophilic Substitution Reaction (SNi Reaction)
Nucleophilic substitution reaction which follows second-order kinetics and yet with no change in the configuration of the product is identified as SNi reaction. The rate of the reaction is found to be dependent on both the substrate and reagent.
Rate ∝ [Substrate][Reagent]
In SNi reaction, part of the leaving group acts as a nucleophile. A typical example of this mechanism is the chlorination of alcohols with thionyl chloride.

Reaction takes place in two steps. First step is heterolytic fission and formation of an intimate ion pair of carbocation and leaving group.
In the second step, an internal nucleophilic which is part of the leaving group attacks from the front side results in the formation of product with retention of configuration.
Mechanism of SNi Reaction
SOCl2 react first with alcohol to give alkyl chloro sulphite followed by loss of an SO2 molecule and its displacement by its own chloride ion.

When the reaction is carried out in the presence of pyridine, the pyridine hydrochloride formed in the reaction supplies the effective nucleophile, Cl−, for a back-side attack as in normal SN2 reaction with inversion of configuration.