Cleansing Action of Detergent
Cleansing action of detergents is due to their capacity to reduce the surface tension of water, emulsify oil or grease, and retain it in suspension in water.
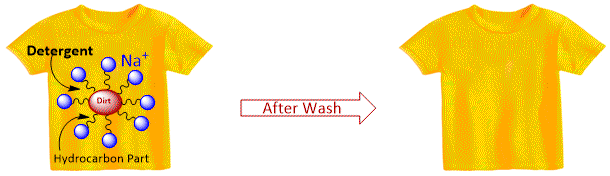
Synthetic detergent have the same type of molecular structure as soap i.e. a tadpole like molecule having two parts. First part is a long chain hydrocarbon part (hydrophobic, insoluble in water but soluble in oil and grease) and second part is a short ionic part SO3Na or SO4Na (hydrophilic, soluble in water but insoluble in oil and grease). Thus the cleansing action is exactly similar to that of soaps whereby the formation of micelles followed by emulsification occurs. The only difference is that detergents work with hard water also.
When the detergent is added to dirty clothes having grease and oily substances, the greasy and oily dirt particles attach themselves to the hydrocarbon part and ionic part remains attached to the water. When the dirty clothes are agitated in a detergent solution, the dirt particles attached to the hydrocarbon part molecule get washed away in water and the clothes get cleaned.
However synthetic detergents can lather well even in hard water because they are soluble in sodium or potassium salts of sulphonic acid or alkyl hydrogen sulphate and similarly form soluble calcium or magnesium salts on reacting with the calcium ions or magnesium ions present in water. This is a major advantage of the cleansing property of detergents over soap.
