Beer-Lambert Law
The Beer-Lambert law (or Beer's law) is the linear relationship between absorbance and concentration of an absorbing species. The general Beer-Lambert law is usually written as:
A = λ . x . c
where A is the measured absorbance, λ is a wavelength-dependent absorptivity coefficient, b is the path length, and c is the analyte concentration.
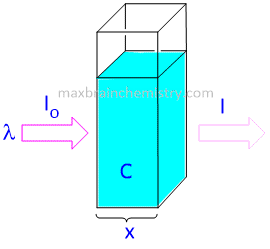
When working in concentration units of molarity, the Beer-Lambert law is written as:
A = ε . x . c
where epsilon(ε) is the wavelength-dependent molar absorptivity coefficient with units of M-1 cm-1.
For details click here
MCQs Bases on Beer-Lambert Law
1. Which of the following equations correctly represents the Beer-Lambert Law?
A. A = ϵ c l
B. A = ϵ + c + l
C. A = ϵ c l
D. A = ϵ c / l
View Answer
Option A is correct answer.
A = ϵ c l.
2. According to the Beer-Lambert Law, absorbance (A) is directly proportional to which of the following?
A. Only the intensity of incident light
B. Only the path length
C. Both the concentration of the solution and the path length
D. Only the wavelength of light
View Answer
Option C is correct answer.
Both the concentration of the solution and the path length.
3. If the absorbance of a solution is 1 at a specific wavelength, what percentage of the incident light is transmitted through the solution?
A. 100%
B. 10%
C. 1%
D. 0%
View Answer
Option B is correct answer.
10%.
A = −log10T
or, 1 = −log10T
Multiply both sides by -1, we get
−1 = log10T
or, T = 10−1
or, T = 0.1
So, Percentage Transmittance = T × 100%
Percentage Transmittance = 0.1 × 100%
Percentage Transmittance = 10%
4. What is the relationship between absorbance (A) and transmittance (T) in the context of the Beer-Lambert Law?
A. A = log10T
B. A = −log10T
C. A = T2
D. A = 1/T
View Answer
Option A is correct answer.
A = log10T.
5. Which of the following is NOT an assumption or condition for the Beer-Lambert Law to be valid?
A. The solution must be homogeneous
B. The incident light must be monochromatic
C. The solute should not undergo association or dissociation
D. The solution must be highly concentrated
View Answer
Option D is correct answer.
The solution must be highly concentrated.
6. What happens to the absorbance if the path length through the solution is doubled, assuming concentration and molar absorptivity remain constant?
A. Absorbance is halved Option C is correct answer. The Lambert-Beer Law is expressed by the equation:
B. Absorbance remains unchanged
C. Absorbance doubles
D. Absorbance becomes zero
View Answer
Absorbance doubles.
A = ϵ b c
This equation shows a direct proportionality between absorbance (A) and path length (b). If the path length is doubled (2b), and ϵ and c remain constant, then the new absorbance (A′) would be:
A′ = ϵ(2b)c
A′ = 2(ϵbc)
A′ = 2A
Therefore, if the path length is doubled, the absorbance also doubles.
7. In spectrophotometry, the path length (b) is typically determined by
A. The concentration of the analyte.
B. The wavelength of incident light.
C. The dimensions of the cuvette or sample cell.
D. The molar absorptivity of the substance.
View Answer
Option C is correct answer.
The dimensions of the cuvette or sample cell.
8. If 50% of the incident light is absorbed by a sample, what is its approximate absorbance (A)?
A. 0.301
B. 0.500
C. 0.693
D. 1.000
View Answer
Option C is correct answer.
Since 50% absorbed means 50% transmitted (T = 0.5).
A = −log(0.5) ≈ 0.301.
9. Which of the following fundamental principles forms the basis of the Lambert-Beer Law?
A. The direct proportionality between transmittance and concentration.
B. The exponential decrease in light intensity with increasing path length.
C. The inverse relationship between absorbance and incident light intensity.
D. The constant absorption of light regardless of the medium.
View Answer
Option A is correct answer.
The direct proportionality between transmittance and concentration.
10. A limitation of the Lambert-Beer Law at high concentrations is due to
A. Increased solvent evaporation.
B. Changes in the refractive index of the solution.
C. Decreased scattering of light by the solution.
D. Formation of aggregates or non-ideal behaviour of molecules.
View Answer
Option D is correct answer.
Formation of aggregates or non-ideal behaviour of molecules
At high concentrations, molecules are closer together, leading to interactions that affect how they absorb light. Additionally, the refractive index of the solution changes with concentration.
