Beer-Lambert Law
The Beer-Lambert law (or Beer's law) is the linear relationship between absorbance and concentration of an absorbing species. The general Beer-Lambert law is usually written as:
A = λ . x . c
where A is the measured absorbance, λ is a wavelength-dependent absorptivity coefficient, b is the path length, and c is the analyte concentration.
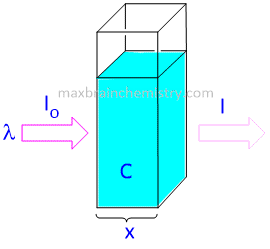
When working in concentration units of molarity, the Beer-Lambert law is written as:
A = ε . x . c
where epsilon(ε) is the wavelength-dependent molar absorptivity coefficient with units of M-1 cm-1.
For details click here
