Chemistry of Ethers and Epoxides
Ethers are organic compounds with an oxygen atom between two carbon groups (R-O-R'), while epoxides are cyclic ethers with a three-membered ring containing one oxygen and two carbon atoms, making them highly reactive due to ring strain. Epoxides are also known as oxiranes. Ethers may be symmetric or asymmetric. The simplest and the most important epoxide is ethylene oxide.
Nomenclature of Ethers
Common Names: Name the two alkyl or aryl groups attached to the oxygen atom alphabetically, followed by the word ether. Example: CH3-O-CH2-CH3 is ethyl methyl ether.
IUPAC Names: The larger alkyl or aryl group is considered the parent chain, and the smaller group with the oxygen is named as an alkoxy substituent. Example: CH3-O-CH2-CH3 is methoxyethane. For more complex ethers, systematic IUPAC names are used based on the parent chain and alkoxy substituents.
Methods of Preparation of Ethers
Williamson Ether Synthesis
It is an important laboratory method for the preparation of symmetrical and unsymmetrical ethers. In this method, an alkyl halide is allowed to react with sodium alkoxide.
R–X + R–ONa → R–O–R + Na–X
Ethers containing substituted alkyl groups (secondary or tertiary) may also be prepared by this method. The reaction involves SN2 attack of an alkoxide ion on primary alkyl halide.
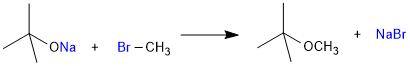
Better results are obtained if the alkyl halide is primary. In case of secondary and tertiary alkyl halides, elimination competes over substitution. If a tertiary alkyl halide is used, an alkene is the only reaction product and no ether is formed. For example, the reaction of CH3ONa with (CH3)3C–Br gives exclusively 2-methylpropene

It is because alkoxides are not only nucleophiles but a strong bases as well. They react with alkyl halides leading to elimination reactions.
By Dehydration of Alcohols
Alcohols undergo dehydration in the presence of protic acids (H2SO4, H3PO4) to form ethers at 443K.
C2H5–OH → C2H5–O–C2H5
The method is suitable for the preparation of ethers having primary alkyl groups only otherwise the reaction favours the formation of alkene by follows SN1 pathway.
Synthesis of Methoxy Ethers
Methoxy ethers can be easily prepared by the reaction of alcohol with diazomethane in the presence of HBF4.
R–OH + CH2N2 → R–O–CH3 + N2↑
Physical Properties of Ethers
◉ Dimethyl ether and diethyl ether are solids and rest are liquids. Some aromatic ethers are solid.
◉ Ethers have a lower boiling points than their corresponding isomeric alcohols, as they do not from hydrogen bond like alcohols. For example,
C2H5OH > CH3–O–CH3.
◉ Ethers are partially soluble in water due to the formation of hydrogen bonds with water.
◉ Ethers are weak Bronsted bases or Lewis bases, as the central atom oxygen has 2 lone pair of electrons to donate and can also accept H+
ions.
◉ Due to presence of lone pair of electrons on oxygen atom, ethers have some value of dipole moment.
Chemical Properties of Ethers
Cleavage of C–O Bond in Ethers
Ethers are the least reactive like alkanes. The cleavage of C-O bond in ethers takes place under drastic conditions with excess
of hydrogen halides. The reaction of dialkyl ether gives two alkyl halide molecules. The reaction proceeds via an SN1 or SN2 mechanism, depending on the structure of the ether.
R–O–R + H–X → R–X + R–OH
R–OH + H–X → R–X + H2O
Ethers with two different alkyl groups are also cleaved in the same manner.
R–O–R + H–X → R–X + R–OH
The order of reactivity of hydrogen halides is as follows:
HI > HBr > HCl.
The cleavage of ethers takes place with concentrated HI or HBr at high temperature.
When one of the alkyl group is a tertiary group, the halide formed is a tertiary halide because, in step 2 of the reaction, the departure of leaving group (HO–CH3) creates a more stable carbocation [(CH3)3C+], and the reaction follows SN1 mechanism.

In case of anisole, methylphenyl oxonium ion, is formed by protonation of ether. The bond between O–CH3 is weaker than the bond between O–C6H5 because the carbon of phenyl group is sp2 hybridised and there is a partial double bond character. Therefore, the attack by I– ion breaks O–CH3 bond to form CH3I. Phenols do not react further to give halides because, the sp2 hybridised carbon of phenol cannot undergo nucleophilic substitution reaction needed for conversion to the halide.

Alkyl aryl ethers are cleaved at the alkyl-oxygen bond due to the more stable aryl-oxygen bond. The reaction yields phenol and alkyl halide but not aryl halide and alkyl alcohol.

Autoxidation
Ethers can react with oxygen from the air to form explosive hydroperoxides and peroxides. This is a slow process but can be hazardous, especially with prolonged storage. Autoxidation of ethers occurs through a free-radical chain reaction.

Ziesel's Method
Ziesel's Method is used to determine the number of methoxy (-OCH3) groups in a compound. The compound is heated with HI, releasing CH3I, which is then reacted with AgNO3 to form AgI precipitate. The weight of AgI is used to calculate the number of methoxy groups.
R-O-CH3 + HI → ROH + CH3I
CH3I + AgNO3 → AgI (precipitate) + CH3NO3
Electrophilic Substitution Reactions
The alkoxy group (-OR) is ortho, para directing and activates the aromatic ring towards electrophilic substitution in the same way as in phenol.
Halogenation of Anisole
Anisole undergoes bromination with bromine in ethanoic acid even in the absence of iron (III) bromide catalyst due to the activation of benzene ring by the methoxy group. Para isomer is obtained in 90% yield.

Friedel-Crafts Reaction of Anisole
Anisole undergoes Friedel-Crafts reaction, i.e., the alkyl and acyl groups are introduced at ortho and para positions by reaction with alkyl halide and acyl halide in the presence of anhydrous aluminium chloride (a Lewis acid) as catalyst.

Nitration of Anisole
Anisole reacts with a mixture of concentrated sulphuric and nitric acids to yield a mixture of ortho and para nitroanisole.

Reaction with Carbonmonoxide
Ethers reacts with CO to form esters in BF3 at 150° under pressure.
R–O–R + CO → R–CO–O–R
Reaction with PCl5
Ethers on heating with PCl5 gives alkyl chloride.
R–O–R + PCl5 → 2R–Cl + 2POCl3
Nomenclature of Epoxides
Common Names: Common names are derived from the name of the alkene from which the epoxide is derived and are named as alkeneoxide.
IUPAC Names: Simple epoxides are named as derivatives of oxirane or by prefixing alkane with epoxy.
Methods of Preparation of Epoxides
Reaction of Alkenes with Peroxyacids
Alkene reacts with peroxyacids such as m-chloroperoxybenzoic acid (mCPBA), peracetic acid, or perbenzoic acid in an inert solvent like dichlorometane, carbon tetrachloride etc, gives epoxide. Peracids acts as oxidizing agents. The reaction is known as epoxidation of alkene and is stereospecific that means cis-alkenes give cis-epoxides (also called syn addition), and trans-alkenes give trans-epoxides.

From Halohydrins
When halohydrins are treated with a base like NaOH or KOH, epoxide is formed with the elimination of HX.

Physical Properties of Epoxides
◉ Lower molecular weight epoxides are volatile liquids at room temperature.
◉ Most epoxides are colorless.
◉ Epoxides are slightly polar due to the presence of the oxygen atom.
◉ Smaller epoxides are somewhat soluble in water due to their slight polarity while larger epoxides are less water-soluble but soluble in organic solvents.
◉ The boiling points of epoxides are generally lower than those of alcohols with the same number of carbon atoms.
◉ The densities of epoxides are usually close to that of water.
Chemical Properties of Epoxides
Acid Catalysed Ring Opening
When an epoxide is treated with a nucleophile (H2O, R-OH, HX, HCN, MeSH etc.) in the presence of an acid, C-O cleavage. In unsymmetrical epoxides the bond cleavage takes place in such a way that most stable carbocation is formed.

Base Catalysed Ring Opening
In the presence of a base like sodium alkoxides, ammonia/amines, nucleophilic attack takes place at less sterically hindered side.

Molecular rearrangement
Ethylene oxide undergoes molecular rearrangement on heating to form acetaldehyde.

Reaction with Grignard reagents and organolithium Reagents
Ethylene oxide reacts with Grignard reagent and organolithium reagents to form addition products which on acid hydrolysis give primary alcohols. The primary alcohol thus produced having two carbon more than the starting reagent.

Grignard and organolithium reagents attack epoxides at the least hindered carbon to generate alcohols.

Reduction with Lithium Aluminium Hydride
Epoxide reduced to alcohol by Lithium Aluminium Hydride (LiAlH4)

Ethers and Epoxides MCQs
50 Objective Questions
B.Sc. 4th Semester
Your Test Result
You answered out of questions correctly.
You got % marks.

